
You may have heard that the FDA ruled in 2023 that trans fats are not safe and can no longer be added to food. While this is certainly a step in the right direction, the trans fat ban left food manufacturers scrambling for another ingredient that will provide the same yummy flavor and long shelf life as trans fats. They found one—a sneaky unregulated form of trans fat known as monoglycerides and diglycerides.
How is this possible? The FDA only recognizes fats that are in the form of a triglyceride as dietary fats. Fats that are in the mono- and diglyceride form are not recognized as dietary fats (even though they are fats obtained through our diet!). Instead, they are classified as food additives called emulsifiers. Thus, the FDA ban on trans fats doesn’t apply to mono- and diglycerides.
Before I explain what mono-and diglycerides are, and why you should beware of them in your food, I want to provide a refresher on what trans fats are, and why they’ve finally been banned….
What is Trans Fat?
Trans fats were born in 1901 when Wilhelm Norman, a German chemist, patented the chemical process for changing liquid oils into solid fats. The process involves bubbling hydrogen into natural oils, causing rearrangements in the structures and chemical properties of the fat molecules. This process, called “hydrogenation”, creates fat molecules with chemical structures that do not exist in nature. The food industry dubbed them “hydrogenated oils.”
This newfangled man-made fat was not only cheap to produce, but it had the same molecular stability, yummy flavor, and long shelf life as the natural saturated fats it was designed to replace—namely butter, coconut oil, and lard. The first trans fat product, Crisco, was introduced in grocery stores in 1911. During the Second World War, butter rationing created a demand for margarine made from trans fats. By the late 1980s, nearly all saturated fats in commercial food products had been replaced by trans fats made from soybean oil.
How Trans Fats are Made
To create trans fats, manufacturers start with inexpensive oils—such as soy, corn, cottonseed, or canola—which are often already rancid from the extraction process. These oils are combined with a metal catalyst, typically tiny particles of nickel oxide, and then exposed to hydrogen gas in a high-pressure, high-temperature reactor. To improve consistency, starch and soap-like substances called emulsifiers are added, followed by steam-cleaning at high temperatures to eliminate the oil’s unpleasant odor. At this stage, the oil takes on an unappealing gray color (unbecoming of anything we’d want to spread on our toast), which is then bleached to appear more appetizing (think Crisco). To mimic the look and taste of butter, manufacturers add dyes and artificial flavors—so convincingly that products like I Can’t Believe It’s Not Butter! were born. Finally, the processed mixture is compressed and packaged into tubs or blocks, long marketed as a “healthier” alternative to real butter.
Why Trans Fats Are Bad for Your Health
Up until the 1970’s, trans fat was considered a cheaper alternative to butter, coconut oil and lard, and some health groups promoted partially hydrogenated oil as beneficial because it was thought to be safe and healthier than animal fats and tropical oils.
The first evidence of concern about the safety of trans fats emerged in the 1980s. In the early 1990s, research began highlighting the deleterious health effects of trans fats, and by the mid-2000s, strong evidence confirmed their role in diabetes, obesity, inflammation and plaque buildup in the arteries leading to coronary heart disease, likely due to their adverse impact on insulin resistance and elevating LDL (“bad”) cholesterol levels. In response, Denmark became the first country to ban partially hydrogenated oils in 2003, paving the way for similar actions in other nations. In the United States, New York City implemented a ban on trans fats in restaurant foods in 2006, followed by the state of California imposing a similar ban on trans fats in restaurant foods in 2008.
in 2015 the FDA finally acted on what scientists at the National Academy of Sciences had declared nearly 15 years prior—that trans fats increase the risk of heart disease and there is no safe level of trans fats—and revoked their “Generally Recognized as Safe” (GRAS) status. The FDA specifically ruled that trans fat was no longer considered safe and could no longer be added to food after June 18, 2018, unless a food manufacturer could present convincing scientific evidence that a particular use was safe.
But when that date arrived, the food manufacturers requested more time, so the FDA gave them until January 1, 2021, as the final compliance date to reformulate their food products and ensure a “smooth transition” in the marketplace. And just when we thought the saga was over, the FDA announced that December 22, 2023, would be the effective date for the direct final rule regarding the revocation of uses of trans fats in food.
So yeah, it took the FDA a minute to finally ban trans fats, but they haven’t gone far enough. Food manufacturers have a loophole that allows certain trans fat-containing compounds called mono- and diglycerides—to remain unregulated in our food.
Understanding the Molecular Structure of Triglycerides, Diglycerides and Monoglycerides
To grasp the distinction between triglycerides, monoglycerides, and diglycerides, we must first understand their molecular structures.
The vast majority of dietary fats are in the form of a triglyceride, which is nature’s way of bundling three individual fatty acid molecules together into one larger molecule. Triglycerides are found in nuts, seeds, whole grains, oils pressed from nuts, seeds, and grains, as well as in foods like olives, avocados, palm fruit, egg yolks, and animal fats.
The prefix “tri” means “three”, so the molecular structure of triglycerides consists of three fatty acids attached to a glycerol molecule.
The glycerol portion of the molecule is called the backbone, and from each of its three carbon atoms hangs a free-swinging fatty acid, making a molecule that looks like a three-pronged fork.
Another way to think of the molecular structure of a triglyceride is that it looks like three fingers attached to three knuckles. If you fold your thumb and pinky finger under the palm of your hand and hold out your three middle fingers, you can get an idea of what a triglyceride looks like. The knuckles are like the three carbon atoms in the glycerol backbone and the three fingers are like the individual fatty acid tails.
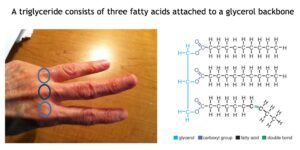
The fatty acids in the triglyceride can be either saturated or unsaturated. Saturated fats have no double bonds. Unsaturated fats have one to six double bonds within the molecule. Unsaturated fats can be either cis fats or trans fats.
The Latin prefixes cis and trans describe the orientation of the hydrogen atoms with respect to the double bond. Cis means “on the same side” and trans means “across” or “on the other side.” The cis configuration is the one that generally occurs in nature. It forms a “bend” in the molecule, and that’s what makes oils liquid at room temperature.
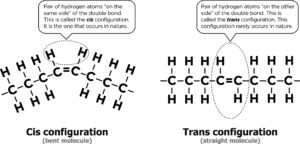
The cis configuration can only be changed through a forced chemical reaction that breaks the double bond and rearranges the hydrogen atoms. This forced chemical reaction is called hydrogenation. Under high temperatures, the nickel catalyst causes the hydrogen atoms to change position at the double bond: one hydrogen atom of the pair is moved to the other side so that the molecule straightens. This is called the trans configuration, a molecular structure rarely found in nature. Because of this change in configuration, the chemical properties of the trans fat are quite different from the chemical properties of the naturally occurring cis fat. For one, it changes from a liquid to a solid, but it also does not work in our body the same way, which is why trans fats are harmful to our health.
Now that you know what a triglyceride looks like, imagine what it would look like if one of the fatty acids on either the top or bottom of the molecule were removed. Using your fingers as a model again, you can fold under either the index finger or the ring finger, and now you only have two fingers sticking out. That is what a diglyceride looks like.
Diglycerides contain a glycerol backbone with two fatty acids attached. The prefix “di” means “two”, thus the name. If one or both of those fatty acids is a cis fat, the diglyceride is healthy. But if one or both of those fatty acids is a trans fat, the diglyceride is bad news for the body.
Now imagine that both the top and bottom fatty acids are removed from the triglyceride, and only the middle one remains attached to the glycerol backbone. This is called a monoglyceride. The prefix “mono” means “one”, thus the name. If that fatty acid is a cis fat, the monoglyceride is healthy. But if it is a trans fat, the monoglyceride is bad news for the body.
Both monoglycerides and diglycerides can be created during the breakdown of triglycerides through industrial processing. However, unlike triglycerides, they are not considered major dietary fats.
Why Aren’t Mono- and Diglycerides Regulated as Fats?
The key reason mono- and diglycerides escape trans fat regulations is classification. The FDA and other food regulatory agencies categorize these compounds as emulsifiers rather than dietary fats. Emulsifiers are added to foods to help keep water and oil from separating. They are also used to extend the shelf life of products. Mono and diglycerides are industrially produced from vegetable oils (such as soybean, grapeseed, canola, sunflower, cottonseed, coconut, and palm oil) and plant pomace such as grape pomace or tomato pomace, although animal fats are sometimes used so if you’re a vegan you should definitely be aware of them in your food products.
Since they technically do not fit the definition of triglycerides, they bypass the rules that apply to traditional fats, including the ban on artificial trans fats.
This classification as emulsifiers means:
• They do not contribute to the total fat content on nutrition labels.
• They are not counted towards trans fat limits, even if they contain trans fat residues.
• They appear as “emulsifiers” or “mono- and diglycerides” on ingredient lists, rather than being labeled as hydrogenated fats.
Why Are Mono- and Diglycerides Used in Foods?
Food manufacturers rely on mono- and diglycerides for their emulsifying properties, which help mix ingredients that would otherwise separate, such as oil and water. They improve texture, shelf life, and stability in various processed foods, including:
• Baked goods (bread, cakes, cookies) – to improve dough texture and prevent staleness.
• Margarine and spreads – to create a smooth, spreadable consistency.
• Ice cream and dairy products – to maintain a creamy texture and prevent ice crystals.
• Infant formula – to maintain a creamy texture.
• Jams and jellies – to create a smooth, spreadable consistency.
• ReddiWhip whipped cream- to maintain a creamy texture.
• Coffee creamer, like Coffeemate- to maintain a creamy texture.
• Chocolate, peanut butter and nut spreads – to prevent oil separation.
• Processed and fast foods – to enhance moisture retention and mouthfeel.
• A product called Apeel, which is a plant-based coating that’s applied to fruits and vegetables to help them stay fresh longer.
How to Avoid Mono- and Diglycerides in Your Diet
Reducing exposure to hidden trans fats requires careful label reading and opting for whole, unprocessed foods. Here are some tips:
• Read ingredient lists carefully – Look for terms like “mono- and diglycerides,” “distilled monoglycerides,” or “partially hydrogenated emulsifiers.”
• Choose whole foods over processed foods – Fresh fruits, vegetables, whole grains, and unprocessed meats do not contain these additives.
• Select organic or minimally processed baked goods – Many organic products use natural emulsifiers like lecithin instead.
• Make homemade versions of common processed foods – Baking your own bread or making nut butter at home eliminates the need for synthetic emulsifiers.
• Opt for brands that use clean ingredients – Some brands specifically avoid artificial emulsifiers in their products.
While trans fats have been largely banned from processed foods, mono- and diglycerides serve as a loophole allowing trace amounts of these harmful fats to persist in our food supply. Due to their classification as emulsifiers, they are exempt from fat labeling regulations, making it harder for consumers to track their intake. Being aware of their presence in processed foods and making informed choices can help reduce your exposure to hidden trans fats and promote better health.
References:
Final determination regarding partially hydrogenated oils. (2015, June 17). Federal Register. https://www.federalregister.gov/documents/2015/06/17/2015-14883/final-determination-regarding-partially-hydrogenated-oils
Kalmus, S. (n.d.). What is bad about mono- & diglycerides? Livestrong.com. https://www.livestrong.com/article/445850-what-is-bad-about-mono-diglycerides/
Mozaffarian, D., Katan, M. B., Ascherio, A., Stampfer, M. J., & Willett, W. C. (2006). Trans fatty acids and cardiovascular disease. New England Journal of Medicine, 354(15), 1601-1613. https://doi.org/10.1056/nejmra054035
National Academy of Sciences. (2002). Report offers new eating and physical activity targets to reduce chronic disease risk. News from the National Academies. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=10490
Sellem, L., Srour, B., Javaux, G., Chazelas, E., Chassaing, B., Viennois, É., Debras, C., Salamé, C., Druesne-Pecollo, N., Esseddik, Y., De Edelenyi, F. S., Agaësse, C., De Sa, A., Lutchia, R., Louveau, E., Huybrechts, I., Pierre, F. H., Coumoul, X., Fezeu, L. K., . . . Touvier, M. (2023). Food additive emulsifiers and risk of cardiovascular disease in the NutriNet-Santé cohort: Prospective cohort study. BMJ, 382, e076058. https://doi.org/10.1136/bmj-2023-076058

